Automated Robotic Polymerase Chain Reaction (PCR) Testing System Installed at Kansai International Airport
May 20, 2021

Tokyo, May 20, 2021 – Kawasaki Heavy Industries, Ltd. announced today that it has installed an automated robotic PCR testing system (hereinafter “the System”) at the entrance to the international departure terminal located on the fourth floor of Kansai International Airport (KIX), in preparation for the planned launch of Japan’s first airport PCR testing service for passengers taking outbound flights.
The global expansion of COVID-19 has severely limited peoples’ activities, resulting in a significant reduction in the number of airport users. To achieve an economic recovery in such an environment, increasing the use of airports-our transportation hubs-is an urgent matter to which Japanese and other governments, as well as industries across the world, are paying close attention.
The System, which was jointly developed by Kawasaki, Sysmex Corporation, and Medicaroid Corporation, is capable of testing up to 2,500 specimens per day in a fully-automated manner. Its efficacy was proven in a joint research project between Kawasaki and Fujita Health University (located in Aichi Prefecture), and the System went into use in March 2021, at the university’s testing facility.
At KIX, the System will be able to provide PCR testing not only to passengers, but also to those who work at the airport and related facilities. In the future, if it is deployed at a variety of locations, it will benefit essential workers such as nursing home staff and healthcare professionals, and participants in local governments’ tests conducted to monitor the rate of infection. The System is expected to contribute to achieving both effective measures against the pandemic and the sustenance of socioeconomic activities.
Kawasaki will collaborate with Kansai Airports Co., Ltd., which operates KIX, to implement a full-scale testing service.
Advantages and Overview of the System
1) Advantages
- Uses a real-time reverse transcription (RT) PCR method (regarded as the global standard), achieving reduced testing time (approx. 80 minutes)
- With robotic automation, it achieves high throughput, excellent accuracy, and stable testing, reducing the burden on physicians and other healthcare professionals
- Achieves safety, simplified operations, and a reduction in labor with its remote monitoring
- Its robotic high-throughput testing is in line with a testing method recommended by the Ministry of Health, Welfare and Labour and medical associations
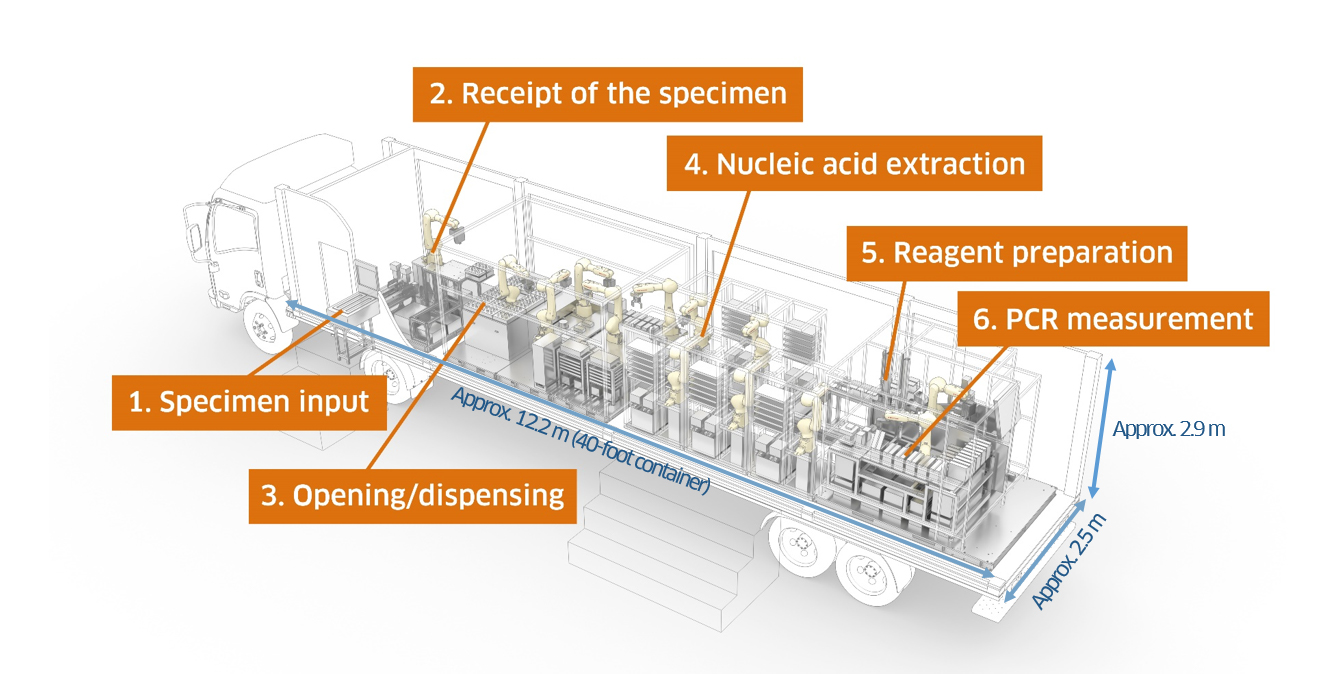
- Saves space (the System fits in a 40-foot container)
- Containerized, it is highly mobile and can be deployed to wide-spread events
- Dimensions: 12.2 m (L) x 2.5 m (W) x 2.9 m (H) (size of the System)
- Throughput: Up to 2,500 specimens/day (when operating 16 hours/day)
2) Overview
Contact
If you need more information about our business,
please feel free to contact us.





